Abstract
Cytomegalovirus (CMV) infection remains a major cause of morbidity/mortality after allogeneic hematopoietic cell transplantation (HCT). Preemptive antiviral therapy is associated with drug-induced toxicities, and prophylactic therapy with letermovir is associated with late reactivations and delayed antiviral immune reconstitution. Therefore, substituting antivirals with a vaccine that harnesses the native immune response to CMV may improve outcomes for HCT recipients. Our group has developed a peptide vaccine, CMVPepvax, composed of an HLA-A*0201 restricted pp65 495-503 CD8 T cell epitope, covalently linked to a universal tetanus T helper epitope and co-administered with PF-03512676 adjuvant. CMV PepVax was safe and immunogenic in a healthy volunteer study (La Rosa et al. PMID: 22402037;) and a phase Ib HCT recipient trial (Nakamura, et al. PMID: 26853648) with the latter demonstrating a promising sign of efficacy in reducing CMV viremia.
In this double blind, placebo-controlled, randomized phase 2 trial (NCT02396134), HCT recipients were enrolled at four USA transplant centers. Eligible patients were CMV seropositive, HLA A*0201-positive, 18-75 years, receiving HCT from a matched related/unrelated donor. T-cell depleting agents (i.e. ATG) or recipients of ex-vivo T-cell-depleted grafts were excluded. Prophylactic antiviral therapy was not allowed. Patients were enrolled prior to day 0 of HCT and reassessed on day +28 for eligibility and randomization to the vaccine (VA) or placebo arm (PA), stratified by donor CMV serostatus. PepVax was administered subcutaneously on days +28 and +56 post-HCT. The primary endpoint of the trial was CMV viremia ≥1250 IU/m or CMV disease through 100 days post-HCT. A total of 96 patients were planned to be randomized at 1:1 ratio, providing 90% power to detect a reduction of viremia from 40% to 15%. CMVpp65-specific immune reconstitution was monitored by measuring levels of CD8 T cells binding to MHC class I pp65 495-503 and HIVgag 77-85 (as control) multimers (Immudex Dextramers), as well as CD28 and CD45 memory markers (BD Biosciences). The intensity of the fluorescent labels was measured using a Gallios flow cytometer with Kaluza software (Beckman Coulter).
Enrollment started in June 2015 but was stopped in November 2017 when a planned interim analysis suggested futility for the primary efficacy endpoint. By that time, 76 subjects had been consented, of whom 61 met the day 28 eligibility criteria and were randomized to the VA (n=32) or PA (n=29). The unblinded data were released when the planned one-year follow up was completed for these 61 subjects. The two groups were overall balanced in their demographics and HCT characteristics. There was no difference in the primary endpoint of CMV reactivation/disease between VA (25.1%) and PA (13.8%, p=0.15). The incidence of preemptive therapy was similar between the two arms. PepVax was well tolerated with no increase in adverse events. Transplant outcomes were also similar between the two groups with regards to 1-year overall survival, relapse-free survival, non-relapse mortality, relapse, and acute GVHD. In subjects in VA who reached the primary endpoint (n=8), CMV viremia occurred at a median of 20 days (interquartile range: 15-23 days; range, 0-48) after the first vaccine, suggesting that there was insufficient time for the vaccine-induced T cell expansion.
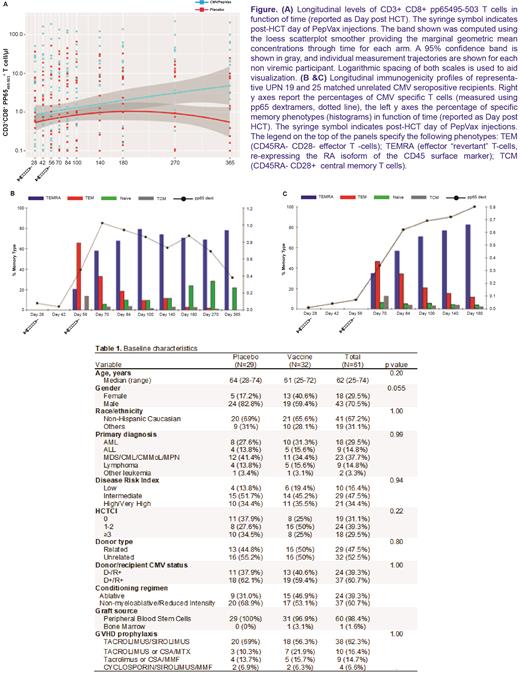
Significantly higher levels of long lasting pp65-specific T cells with effector memory phenotype were measured in non viremic participants in the VA compared to those in the PA; this effect was driven by differences observed late after vaccination (p = 0.004 by GEE analysis; Figure, panel A). In patients who controlled viremia, robust expansion of functional pp65-specific CD8 T cells was observed following PepVax injections (Figure, panels B-C).
Our results confirm safety and immunogenicity of PepVax in CMV seropositive HCT recipients. However, the trial failed to demonstrate a clinical efficacy of PepVax in reducing CMV viremia/disease despite favorable CD8 T cell responses. Early CMV reactivation/disease detected before receipt of the second vaccine may have reduced the ability of PepVax to elicit a protective T cell response. Transfer of vaccine-induced immunity through donor CMV immunization combined with recipient booster immunization may overcome this issue and lead to faster immune reconstitution post-HCT.
Hill: Amplyx: Consultancy; OptumHealth: Consultancy; CRISPR therapeutics: Consultancy; Gilead: Consultancy, Research Funding; Allogene therapeutics: Consultancy; Octapharma: Consultancy; Allovir: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; CLS Behring: Consultancy; Karius: Research Funding. Al Malki: Hansa Biopharma: Consultancy; Jazz Pharmaceuticals, Inc.: Consultancy; Neximmune: Consultancy; Rigel Pharma: Consultancy; CareDx: Consultancy. Pullarkat: Amgen, Dova, and Novartis: Consultancy, Honoraria; AbbVie, Amgen, Genentech, Jazz Pharmaceuticals, Novartis, Pfizer, and Servier: Membership on an entity's Board of Directors or advisory committees. Aribi: Seagen: Consultancy. Devine: Tmunity: Current Employment, Research Funding; Magenta Therapeutics: Current Employment, Research Funding; Sanofi: Consultancy, Research Funding; Johnsonand Johnson: Consultancy, Research Funding; Orca Bio: Consultancy, Research Funding; Be the Match: Current Employment; Vor Bio: Research Funding; Kiadis: Consultancy, Research Funding. Verneris: Novartis: Other: advisory board; jazz: Other: advisory board; Fate Therapeutics: Consultancy. Miller: Fate Therapeutics, Inc: Consultancy, Patents & Royalties, Research Funding; GT Biopharma: Consultancy, Patents & Royalties, Research Funding; Vycellix: Consultancy; ONK Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Magenta: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Wugen: Membership on an entity's Board of Directors or advisory committees. Forman: Allogene: Consultancy; Lixte Biotechnology: Consultancy, Current holder of individual stocks in a privately-held company; Mustang Bio: Consultancy, Current holder of individual stocks in a privately-held company. Diamond: Pfizer Inc: Other; Helocyte Inc: Membership on an entity's Board of Directors or advisory committees, Other, Patents & Royalties.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal